Thermodynamics: Mysteries of heat transfer
Introduction:-
In the intricate web of scientific principles governing our universe, few concepts are as fundamental and enigmatic as thermodynamics. At its core lies the study of heat and its transformation into other forms of energy, shaping everything from the behavior of atoms to the dynamics of galaxies. Among its many facets, heat transfer stands out as a cornerstone, offering a window into the intricate dance of energy exchange that underpins the workings of our world.
Imagine a cup of steaming coffee, radiating warmth into the surrounding air on a chilly morning. Or consider the sensation of warmth spreading through your hands as you hold a freshly baked loaf of bread. These everyday experiences are manifestations of the principles of heat transfer at work, albeit on a macroscopic scale.
But delve deeper, and the world of heat transfer reveals a tapestry of phenomena, from the microscopic jostling of molecules to the grand movements of planetary systems. It is a realm where energy flows relentlessly, seeking equilibrium in its eternal quest to balance the scales of temperature.
At its heart, heat transfer embodies the fundamental laws of thermodynamics, a discipline that transcends the boundaries of physics, chemistry, and engineering. From the classical laws of thermodynamics formulated in the 19th century to the cutting-edge research of modern-day scientists, the quest to understand heat transfer has driven innovation and shaped our understanding of the natural world.
Yet, for all its ubiquity, heat transfer remains cloaked in mystery, with many questions still lingering on the frontiers of scientific inquiry. How does heat propagate through different materials? What mechanisms govern the transfer of thermal energy across vast distances in space? These are the puzzles that continue to intrigue and inspire generations of scientists and engineers.
In this series of articles, we will embark on a journey through the labyrinthine realms of heat transfer, exploring its principles, applications, and unanswered questions. From the timeless elegance of Fourier's law to the esoteric realms of quantum thermodynamics, we will delve into the depths of this captivating field, shedding light on its mysteries and unraveling its secrets.
Join us as we venture into the fascinating world of thermodynamics, where the mysteries of heat transfer await discovery and exploration.
Historical Perspective:-
From the flickering flames of ancient hearths to the intricate designs of modern-day heat exchangers, the concept of heat transfer has captivated human curiosity for centuries. As we delve into the historical tapestry of thermodynamics, we uncover a saga of intellectual triumphs, paradigm shifts, and enduring mysteries.
1. Ancient Insights:
The roots of heat transfer can be traced back to civilizations of antiquity. The ancient Greeks pondered over the nature of heat, proposing the existence of a substance called "phlogiston" that flowed from hot bodies to cold ones. However, it wasn't until the Middle Ages that significant strides were made in understanding the mechanisms of heat transfer.
2. Early Experiments:
In the 17th century, pioneering scientists such as Robert Boyle and Robert Hooke conducted groundbreaking experiments on the behavior of gases and the concept of thermal equilibrium. These endeavors laid the groundwork for the formulation of fundamental principles in thermodynamics.
3. The Birth of Thermodynamics:
The 19th century witnessed the emergence of thermodynamics as a distinct scientific discipline. The works of Sadi Carnot, Rudolf Clausius, and Lord Kelvin revolutionized our understanding of heat and its transformation into mechanical work. Carnot's insight into the idealized efficiency of heat engines paved the way for the development of the first and second laws of thermodynamics.
4. The Steam Age:
The Industrial Revolution ushered in an era of unprecedented technological advancement, driven by the harnessing of steam power. Innovators such as James Watt and George Stephenson applied thermodynamic principles to design more efficient engines, laying the foundation for modern transportation and industry.
5. Maxwell's Demon:
In the late 19th century, James Clerk Maxwell introduced the concept of a hypothetical being, now famously known as Maxwell's demon, capable of violating the second law of thermodynamics by selectively allowing hot and cold molecules to pass through a barrier. This thought experiment challenged conventional notions of entropy and continues to spark debate among physicists to this day.
6. Modern Marvels:
The 20th and 21st centuries have witnessed remarkable progress in our mastery of heat transfer phenomena. From the development of sophisticated heat exchangers for energy-efficient processes to the exploration of novel materials with tailored thermal properties, the quest to unlock the mysteries of heat continues unabated.
7. Challenges and Frontiers:
Despite centuries of study, numerous enigmas persist in the realm of heat transfer. The elusive nature of thermal convection in turbulent flows, the intricacies of nanoscale heat transfer, and the quest for materials with ultra-low thermal conductivity are just a few examples of ongoing research endeavors.
Fundamental Laws of Thermodynamics:-
Thermodynamics, the study of energy and its transformations, governs the behavior of heat and its transfer. Among its cornerstone principles are the fundamental laws that underpin the entire field. These laws not only shape our understanding of energy exchange but also unravel the mysteries behind the intricate dance of heat transfer.
1. The Zeroth Law: A Prelude to Equilibrium
Imagine two bodies, each at different temperatures, brought into contact with each other. What happens next? The Zeroth Law of Thermodynamics elegantly answers this question by introducing the concept of temperature and thermal equilibrium. It states that if two systems are in thermal equilibrium with a third system, then they are in equilibrium with each other. In simpler terms, it’s the law of thermal friendship – bodies in contact eventually reach a state where they are as comfortable with each other’s temperature as they are with their own.
2. The First Law: Conservation of Energy
Known as the law of energy conservation, the First Law of Thermodynamics asserts that energy cannot be created or destroyed, only transformed from one form to another. In the context of heat transfer, this law reminds us that heat is a form of energy, and any change in the internal energy of a system is a result of the heat added to it and the work done on it. Picture a cup of steaming coffee cooling down – as it loses heat to the surrounding air, its internal energy decreases, yet the total energy of the universe remains constant.
3. The Second Law: The Arrow of Time
Arguably the most enigmatic of the laws, the Second Law of Thermodynamics introduces the concept of entropy – a measure of the disorder or randomness of a system. It states that in any natural process, the total entropy of the universe tends to increase over time. This law is the reason why hot coffee cools down, why ice melts in a warm room, and why we can never unscramble an egg. It’s the law that governs the irreversible flow of time, leading to a universe where chaos ultimately triumphs.
4. The Third Law: Absolute Zero and the Quest for Order
As we delve deeper into the realm of thermodynamics, we encounter the Third Law, which posits that as the temperature of a system approaches absolute zero (0 Kelvin), the entropy of the system approaches a minimum value. In simpler terms, it’s the law that defines the ultimate chill – a state of perfect order where molecular motion ceases, and entropy reaches its lowest possible value. While absolute zero is a theoretical concept, its implications reverberate throughout the field of physics, guiding our understanding of phase transitions and the behavior of matter at extreme temperatures.
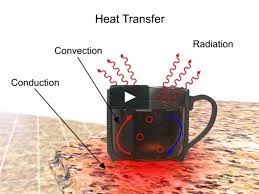
Heat Transfer Mechanisms:-
1. Conduction:-
In the realm of thermodynamics, where energy flows and systems evolve, the phenomenon of conduction stands as a mysterious yet fundamental force governing heat transfer. As we delve into the intricacies of this phenomenon, we uncover a world of microscopic interactions and macroscopic implications, offering insights into the very fabric of our universe.
Conduction, at its core, is the transfer of heat through a material medium, driven by a temperature gradient. It occurs on scales both grand and minuscule, from the warmth felt when touching a sunlit surface to the intricate dance of atoms within a solid lattice. While the concept may seem straightforward, its underlying mechanisms reveal a tapestry of complexity.
At the heart of conduction lies the movement of energy carriers—be it electrons in metals or phonons in non-metallic materials. When a substance is heated at one end, its constituent particles gain kinetic energy, vibrating with greater amplitude. These energetic particles collide with neighboring ones, transferring their energy in the process. This cascade of collisions propagates through the material, leading to a net transfer of heat from hot regions to cooler ones.
However, the journey of heat through a material is not uniform. Factors such as the material's conductivity, geometry, and boundary conditions shape the rate and direction of heat flow. Materials like metals, with their abundance of free electrons, exhibit high thermal conductivity, allowing heat to travel swiftly. In contrast, insulating materials impede the flow of heat, exhibiting thermal resistivity.
The intricacies of conduction become even more apparent when considering the microscopic world. Within a crystalline lattice, atoms oscillate around their equilibrium positions, forming a delicate balance of forces. As heat is introduced, these vibrations intensify, leading to an asymmetry in energy distribution. The transfer of this vibrational energy, mediated by phonons, governs the thermal conductivity of materials and plays a crucial role in phenomena ranging from electronic cooling to thermoelectric power generation.
Yet, despite our understanding of the fundamental principles underlying conduction, mysteries persist. The behavior of materials at nanoscale dimensions challenges conventional models, giving rise to phenomena such as ballistic conduction, where carriers traverse the material without scattering. Similarly, the emergence of exotic materials with unconventional thermal properties, such as thermal metamaterials, defies our traditional notions of heat transfer.
Moreover, conduction intertwines with other modes of heat transfer, such as convection and radiation, in intricate ways. In systems where multiple mechanisms coexist, their interplay can lead to unexpected phenomena, from thermal runaway in electronic devices to the intricate patterns seen in natural convection currents.
As we continue to unravel the mysteries of heat transfer, the study of conduction stands as a testament to the boundless complexity of the physical world. From the microscopic motions of atoms to the macroscopic flow of energy, it offers a window into the intricate dance of particles and fields that underpin our universe. And while many questions remain unanswered, each discovery brings us closer to unlocking the secrets of this timeless phenomenon.
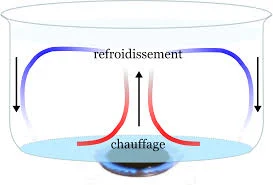
2. Convection:-
In the realm of thermodynamics, where the laws governing the flow of energy dictate the behavior of our universe, convection stands out as a captivating phenomenon. Unlike conduction, where heat travels through a solid medium, or radiation, where energy is transmitted through electromagnetic waves, convection involves the fluid motion of molecules themselves. It's the dance of heat transfer, a ballet of hot and cold currents shaping weather patterns, ocean currents, and even the atmosphere of distant planets.
At its core, convection is a process driven by gradients in temperature and density within a fluid. Picture a pot of water on a stove: as the bottom layer heats up, it becomes less dense and rises, while cooler, denser fluid descends to take its place. This cyclical motion forms the basis of convective currents, transferring heat from one region to another.
But the beauty of convection lies not only in its simplicity but in its complexity. From the swirling eddies of a boiling pot to the majestic updrafts of a thunderstorm, convection manifests in a myriad of forms across scales, from the microscopic to the planetary.
In the kitchen, we witness convection's culinary magic every time we bake a loaf of bread or roast a chicken. Hot air rises in the oven, creating a convection current that evenly cooks our food, imparting that golden crust and succulent interior we crave.
Zoom out, and convection reveals itself as a master sculptor of Earth's climate. Warm air rises at the equator, creating low-pressure systems that drive the trade winds, while cold air sinks at the poles, setting in motion the polar vortex. Ocean currents, too, owe their existence to convection, with warm waters from the equator flowing towards the poles and cold, dense currents returning in a perpetual global conveyor belt.
Yet, despite centuries of study, convection continues to intrigue and mystify scientists. Its chaotic nature defies simple predictions, leading to a rich tapestry of turbulent flow patterns that challenge our understanding. From the meandering path of a river to the sudden formation of a tornado, convection's unpredictable behavior keeps researchers on their toes, driving innovation in fields as diverse as meteorology, engineering, and astrophysics.
Even beyond the confines of our planet, convection shapes the atmospheres of other celestial bodies, from the swirling storms of Jupiter to the molten currents beneath the surface of distant exoplanets. It's a universal principle, governing the dynamics of stars and galaxies, and shaping the very fabric of the cosmos.
As we peer deeper into the mysteries of convection, we uncover not only the secrets of heat transfer but also fundamental truths about the nature of fluid motion and the interconnectedness of our world. It's a reminder that, in the grand symphony of the universe, even the simplest processes can give rise to breathtaking complexity, inviting us to marvel at the beauty and wonder of the natural world.
3. Radiation:-
In the intricate dance of thermodynamics, where heat flows like an invisible river, radiation emerges as a captivating enigma. Unlike conduction and convection, which rely on material mediums, radiation traverses through the vast expanse of space, transcending barriers with ethereal grace. Within this realm of thermal energy exchange, radiation unveils mysteries that have puzzled scientists for centuries.
At its essence, radiation is the transfer of heat in the form of electromagnetic waves, ranging from infrared to ultraviolet and beyond. It's the reason we feel the warmth of the sun on our skin and the chill of a cold night sky. Yet, understanding the intricacies of radiation goes far beyond mere sensation; it delves into the heart of fundamental physical principles.
One of the most intriguing aspects of radiation is its ability to propagate through a vacuum, defying the need for a material medium. This phenomenon puzzled early scientists, challenging conventional wisdom and inspiring groundbreaking discoveries. Through meticulous experimentation and theoretical insights, luminaries such as Max Planck and Albert Einstein laid the foundations for modern quantum mechanics, unraveling the mysteries of radiation's behavior at the atomic level.
The concept of blackbody radiation, where an object absorbs and emits electromagnetic radiation at equilibrium, further deepens the complexity of this phenomenon. Planck's revolutionary quantum hypothesis, proposing that energy is quantized into discrete packets, or quanta, paved the way for a new understanding of radiation. Einstein's elucidation of the photoelectric effect reinforced this quantum framework, establishing the dual nature of light as both particles (photons) and waves.
In the realm of thermodynamics, radiation plays a pivotal role in diverse phenomena, from the greenhouse effect shaping Earth's climate to the thermal radiation emitted by industrial furnaces. Understanding how radiation interacts with matter is essential for optimizing energy efficiency, designing advanced technologies, and exploring the frontiers of space exploration.
Moreover, radiation embodies a captivating duality—it can be harnessed for beneficial applications, such as solar power generation and medical imaging, yet it also poses risks, from the ionizing radiation emitted by nuclear reactions to the harmful ultraviolet rays of the sun.
As we peer into the depths of radiation's mysteries, we encounter phenomena such as the Stefan-Boltzmann law and Wien's displacement law, which elucidate the relationship between an object's temperature and the intensity and wavelength distribution of its emitted radiation. These fundamental principles underpin the design of thermal systems, from spacecraft heat shields to energy-efficient buildings.
In the quest to unlock the secrets of radiation, scientists continue to push the boundaries of knowledge, probing the quantum realm and harnessing advanced computational techniques. From simulating the behavior of plasmas in fusion reactors to modeling the radiative properties of exoplanetary atmospheres, the applications of radiation physics are as diverse as they are profound.
Yet, amidst the complexity and intrigue, one thing remains clear: radiation is not merely a phenomenon to be studied in isolation but an integral part of the rich tapestry of thermodynamics. As we strive to unravel its mysteries, we embark on a journey of discovery, guided by the unyielding curiosity of the human spirit.
Energy Conversion Processes:-
1. Heat Engine:-
In the intricate dance of energy transfer and transformation, few phenomena captivate the human mind like heat engines. These marvels of engineering leverage the fundamental principles of thermodynamics to convert heat into useful work, propelling everything from automobiles to power plants. Delving into the heart of heat engines unveils a world of mystery, where the laws of thermodynamics dictate the boundaries of possibility, and engineers seek to push those limits ever further.
(a) The Essence of Heat Engines:
At their core, heat engines are devices that operate on the principles of thermodynamics to convert thermal energy into mechanical work. They accomplish this feat through a cyclic process, where heat is absorbed from a high-temperature source, transformed into work, and then expelled to a lower-temperature sink. This cycle is governed by the first and second laws of thermodynamics, which establish the conservation of energy and the irreversibility of natural processes, respectively.
(b) The First Law: Conservation of Energy:
The first law of thermodynamics, often referred to as the law of conservation of energy, states that energy cannot be created or destroyed, only transformed from one form to another. In the realm of heat engines, this law manifests as the principle that the total energy input must equal the total energy output, accounting for both the work performed and any heat transferred.
(c) The Second Law: The Arrow of Entropy:
While the first law ensures the conservation of energy, the second law of thermodynamics introduces a concept central to the operation of heat engines: entropy. Entropy is a measure of the disorder or randomness in a system and is fundamentally linked to the directionality of natural processes. In essence, the second law dictates that heat will naturally flow from hot to cold regions, and in doing so, increase the overall entropy of the system.
(d) Carnot's Insight:
In the pursuit of understanding the theoretical limits of heat engines, French engineer Sadi Carnot made a groundbreaking observation in the 19th century. Carnot recognized that the efficiency of a heat engine is determined by the temperatures of the heat source and sink, as well as the nature of the working substance. He formulated the concept of the Carnot cycle, an idealized reversible process that represents the maximum efficiency achievable by any heat engine operating between two temperatures.
(e) Engineering Marvels:
From steam engines to internal combustion engines, heat engines have fueled the industrial revolution and continue to drive innovation in the modern era. Each type of engine employs its own unique configuration and working substance, whether it be water, gas, or even liquid metal. Engineers strive to optimize the efficiency and performance of these machines, often pushing the boundaries of thermodynamic theory through computational modeling and experimental design.
(f) Challenges and Future Directions:
As society grapples with the dual challenges of climate change and finite fossil fuel reserves, the quest for more efficient and sustainable heat engines has taken on renewed urgency. Advances in materials science, renewable energy technologies, and waste heat recovery offer promising avenues for improvement. However, the fundamental constraints imposed by the laws of thermodynamics serve as a constant reminder of the delicate balance between aspiration and reality.
2. Refrigeration Systems:-
In the realm of thermodynamics, the mysteries of heat transfer unfold in the intricate dance of particles within refrigeration systems. From keeping our food fresh to preserving delicate pharmaceuticals, refrigeration plays a vital role in modern society, yet its underlying principles remain shrouded in complexity. Let's embark on a journey to unravel the enigma of heat transfer within refrigeration systems.
At its core, refrigeration is the process of transferring heat from one location to another, typically from a colder environment to a warmer one. This seemingly paradoxical task is accomplished through the manipulation of fundamental thermodynamic principles, primarily those governing the behavior of gases and liquids.
Central to the operation of refrigeration systems is the concept of phase change, wherein a substance transitions between different states, such as from a liquid to a gas or vice versa. This phase change phenomenon lies at the heart of the refrigeration cycle, enabling the extraction of heat from the environment to be cooled.
The refrigeration cycle operates on the principles of compression, condensation, expansion, and evaporation. It begins with the compression of a refrigerant gas, typically a compound such as Freon or ammonia, which increases its temperature and pressure. The hot, pressurized gas then flows through a condenser, where it releases heat to the surrounding environment and condenses into a liquid.
As the liquid refrigerant passes through an expansion valve or device, its pressure decreases dramatically, causing it to evaporate into a gas. This evaporation process absorbs heat from the surroundings, cooling the area in the process. The low-pressure gas is then drawn into the compressor, and the cycle repeats.
One of the key mysteries of heat transfer in refrigeration systems lies in the efficiency of the process. Engineers strive to optimize the system's performance by carefully selecting refrigerants, designing efficient components, and fine-tuning operating parameters. Balancing factors such as energy consumption, environmental impact, and cooling capacity presents a formidable challenge, requiring a delicate equilibrium of scientific understanding and practical engineering.
Moreover, the field of refrigeration continues to evolve as researchers explore innovative technologies and sustainable solutions. From advanced thermoelectric cooling systems to environmentally friendly refrigerants, ongoing efforts seek to push the boundaries of efficiency and sustainability in refrigeration technology.
Yet, amidst the complexities and challenges, the fundamental principles of thermodynamics remain constant, guiding our understanding and shaping the future of refrigeration. As we delve deeper into the mysteries of heat transfer, we unlock new possibilities for cooling and preservation, driving progress and innovation in a world ever reliant on the power of refrigeration.
At the end, the enigma of heat transfer within refrigeration systems serves as a testament to the profound interplay of science, engineering, and innovation. Through the exploration of fundamental thermodynamic principles and the pursuit of technological advancement, we continue to unravel the mysteries of refrigeration, shaping a cooler, more sustainable future for generations to come.
Practical Applications:-
1. Power Generation:-
In the realm of power generation, the intricate dance of thermodynamics governs the transformation of heat into usable energy. At the heart of this process lies the enigmatic concept of heat transfer, where energy flows from hot to cold, driving turbines, and powering our world. Delving into the depths of this phenomenon unveils a world of mysteries waiting to be unraveled.
(a) The Dance of Heat Transfer:
Imagine a coal-fired power plant, where the combustion of fossil fuels releases an intense blaze, heating water to produce steam. This steam, with its boundless energy, surges through turbines, propelling them into motion. But how does this heat transfer occur?
At its core, heat transfer is a tale of three distinct modes: conduction, convection, and radiation. Conduction whispers secrets of molecules passing on their vibrational energy, hand in hand, through solids. Convection reveals the fluid flow of heated particles, carrying warmth from one place to another. Radiation, the silent storyteller, paints a picture of electromagnetic waves dancing through the void, transferring heat across space.
(b) The Mysteries of Efficiency:
As we delve deeper into the world of power generation, the quest for efficiency becomes paramount. Every joule of heat must be harnessed, every degree of temperature difference exploited. Yet, despite our best efforts, inefficiencies linger, shrouded in mystery.
One such enigma lies within the heart of turbines, where frictional forces steal away precious energy. Another mystery lurks in the realm of heat exchangers, where thermal gradients refuse to yield their full potential. Unraveling these mysteries requires a delicate balance of science and ingenuity, pushing the boundaries of what is possible.
(c) The Promise of Renewable Energy:
In recent years, a new chapter has unfolded in the saga of power generation: the rise of renewable energy. Solar panels bask in the radiance of the sun, converting its warmth into electricity. Wind turbines harness the kinetic energy of the breeze, spinning tales of sustainability. Yet, even in this realm of green energy, the mysteries of heat transfer persist.
How do we optimize the capture of solar radiation? Can we enhance the efficiency of wind turbines through innovative designs? These questions linger, beckoning researchers and engineers to unlock the secrets of nature’s bounty.
2. HVAC System:-
In the intricate dance of thermodynamics, where heat is both a comfort and a challenge, HVAC systems stand as guardians, regulating the temperature for our comfort. Behind the scenes, a fascinating interplay of heat transfer mechanisms unfolds, shaping the efficiency and effectiveness of these systems. Let's embark on a journey to demystify the intricate world of heat transfer within HVAC systems.
(a) Conduction: The Silent Traveler of Heat
At the heart of HVAC systems lies the principle of conduction, where heat energy flows through solid materials. Think of it as a relay race, where molecules pass on the baton of thermal energy. In HVAC systems, this occurs within the walls of ducts, pipes, and the structural elements of heating and cooling units. Understanding the conductivity of materials becomes crucial in designing systems that minimize energy loss and optimize heat transfer.
(b) Convection: Stirring the Air for Comfort
Picture a gentle breeze on a hot summer day or the warmth enveloping you near a cozy fireplace. That's convection at play, the process of heat transfer through the movement of fluids, such as air or water. In HVAC systems, convection currents drive the circulation of conditioned air, ensuring uniform temperatures throughout a space. By strategically controlling airflow patterns, designers can enhance comfort and energy efficiency.
(c) Radiation: The Invisible Embrace
While conduction and convection steal the spotlight, radiation quietly shapes our thermal experiences. Unlike the other two mechanisms, radiation doesn't require a medium to propagate. Instead, it emanates directly from surfaces, warming or cooling objects in its path. In HVAC systems, radiant heating and cooling panels offer a subtle yet effective means of maintaining comfort, especially in spaces with high ceilings or large windows.
(d) Phase Change: Transforming States, Transforming Comfort
Enter the realm of phase change, where substances transition between solid, liquid, and gaseous states, unleashing latent heat in the process. HVAC systems harness this phenomenon through refrigeration cycles, where refrigerants undergo controlled phase changes to absorb or release heat. From cooling your home on a scorching day to keeping food fresh in refrigerators, understanding phase change is essential for engineering efficient HVAC solutions.
(e) Challenges and Innovations: Navigating the Complexities
Despite the marvels of heat transfer, HVAC systems face a myriad of challenges, from energy inefficiencies to environmental impacts. Yet, where challenges arise, so do opportunities for innovation. Engineers are exploring novel materials with superior thermal conductivity, developing smart controls that optimize energy usage, and embracing sustainable practices to minimize ecological footprints. As we delve deeper into the mysteries of heat transfer, the quest for greener, more efficient HVAC solutions continues.
3. Renewable Energy:-
Renewable energy holds the promise of a sustainable future, but behind the sleek solar panels and towering wind turbines lies a complex interplay of forces, including the fundamental principles of thermodynamics. At the heart of renewable energy systems is the efficient transfer of heat, a process governed by intricate laws that dictate the flow of energy. In this exploration, we delve into the mysteries of heat transfer within renewable energy technologies, uncovering the mechanisms that drive the conversion of natural resources into usable power.
(a) Harnessing Solar Energy:
Solar energy, abundant and inexhaustible, is one of the cornerstones of renewable energy. At the forefront of solar technology are photovoltaic cells, which convert sunlight directly into electricity. Behind this seemingly straightforward process lies the intricate dance of photons and electrons, where heat transfer plays a crucial role. When sunlight strikes the surface of a solar cell, it is absorbed, leading to an increase in temperature. Efficient heat transfer mechanisms ensure that this thermal energy is dissipated, preventing overheating and optimizing the performance of the cell. Through careful design and engineering, researchers continue to unravel the mysteries of heat transfer in solar energy systems, pushing the boundaries of efficiency and sustainability.
(b) Unleashing the Power of Wind:
Wind energy, another pillar of renewable power, relies on the kinetic energy of moving air to generate electricity. The conversion of wind energy into usable power involves a series of intricate heat transfer processes, from the warming of the Earth's surface by sunlight to the formation of wind patterns driven by temperature differentials. Within wind turbines, heat transfer mechanisms play a critical role in maintaining optimal operating conditions, ensuring that mechanical components remain within safe temperature ranges. From the blades that capture the wind to the gearbox that drives the generator, efficient heat transfer is essential for maximizing the efficiency and longevity of wind energy systems.
(c) Tapping into Geothermal Resources:
Beneath the Earth's surface lies a vast reservoir of heat waiting to be tapped. Geothermal energy harnesses this natural heat through a variety of technologies, including geothermal power plants and heat pumps. The principles of heat transfer are at the core of these systems, as they facilitate the extraction of thermal energy from the Earth and its conversion into usable power. In geothermal power plants, heat transfer mechanisms drive the circulation of fluids through underground reservoirs, where they absorb heat before returning to the surface to drive turbines and generate electricity. Similarly, geothermal heat pumps rely on heat transfer to extract warmth from the ground during winter months and dissipate excess heat during summer, providing efficient heating and cooling solutions for homes and buildings.
Challenges and Future Directions:-
1. Efficiency Enhancement:-
In the realm of thermodynamics, where the laws governing heat, energy, and work intertwine, lies an intriguing puzzle: efficiency enhancement. It's a quest to maximize the useful work output while minimizing energy loss, a pursuit that drives innovation and fuels our progress towards sustainable energy solutions. Let's delve into this captivating topic and uncover the mysteries of heat transfer efficiency enhancement.
Efficiency enhancement in thermodynamics is akin to squeezing every last drop of potential from a system, be it a power plant, an engine, or a refrigeration unit. At its core, it revolves around optimizing the conversion of energy from one form to another, often battling against the relentless march of entropy.
One of the primary avenues for efficiency enhancement is through the improvement of heat transfer mechanisms. Heat transfer, the movement of thermal energy from a hotter object to a cooler one, is fundamental to countless processes in our daily lives and industrial operations. However, enhancing this transfer efficiency is no simple task.
One approach to boosting efficiency is through the optimization of heat exchangers. These devices facilitate the transfer of heat between two or more fluids at different temperatures, such as in power plants, HVAC systems, and refrigeration units. By redesigning the geometry, enhancing the surface area, or improving the fluid dynamics within these exchangers, engineers strive to minimize thermal resistance and maximize heat transfer rates.
Furthermore, advancements in materials science play a crucial role in efficiency enhancement. The quest for better insulating materials to reduce heat loss or more efficient conductors to enhance heat transfer is ongoing. Nanotechnology, for instance, offers promising avenues with its ability to manipulate materials at the molecular level, creating surfaces with tailored thermal properties.
Moreover, the integration of renewable energy sources into existing systems presents both challenges and opportunities for efficiency enhancement. From harnessing solar power to tapping into geothermal energy, each source comes with its unique set of considerations, requiring innovative solutions to optimize efficiency and minimize environmental impact.
In the pursuit of efficiency enhancement, interdisciplinary collaboration is key. Experts from diverse fields such as physics, engineering, materials science, and chemistry converge to tackle this complex problem from multiple angles. From computational simulations to experimental validations, each approach contributes to our understanding and ability to enhance efficiency in thermodynamic systems.
However, amidst all the progress and innovation, there are still mysteries waiting to be unraveled. The elusive quest for absolute efficiency, the theoretical limits dictated by the laws of thermodynamics, serves as a constant reminder of the challenges that lie ahead. Yet, it is precisely these challenges that drive us forward, pushing the boundaries of our knowledge and ingenuity.
As we continue to explore the mysteries of heat transfer and efficiency enhancement in thermodynamics, one thing remains clear: the journey is as important as the destination. Each discovery, each breakthrough, brings us closer to unlocking the full potential of our energy systems and paving the way towards a more sustainable future.
2. Waste Heat Utilization:-
In the labyrinth of thermodynamics, where heat and energy intertwine, lies a realm of untapped potential: waste heat utilization. This subtopic delves into the fascinating domain of harnessing and repurposing heat that would otherwise dissipate into the atmosphere, offering a glimpse into the intricate dance of molecules and the boundless opportunities they present.
At its core, waste heat utilization embodies the fundamental principle of thermodynamics – the conservation of energy. Heat, often considered an undesirable byproduct of industrial processes or mechanical systems, holds within it the latent energy waiting to be harnessed. This energy, if properly captured and redirected, can be transformed into a valuable resource, unlocking a myriad of applications across various sectors.
The journey of waste heat utilization begins with understanding the mechanisms of heat transfer. From conduction to convection, and radiation to phase changes, heat migrates through different mediums, seeking equilibrium. However, in its quest for balance, heat encounters obstacles – barriers that impede its flow and result in excess thermal energy, commonly referred to as waste heat.
Enter the realm of waste heat recovery and utilization, where engineers and scientists embark on a quest to capture and repurpose this untapped energy. Through innovative technologies such as heat exchangers, thermoelectric generators, and Organic Rankine Cycle (ORC) systems, waste heat finds new purpose, driving turbines, generating electricity, or providing heating for industrial processes.
One of the most promising avenues in waste heat utilization lies in the concept of cogeneration, also known as combined heat and power (CHP). This integrated approach maximizes the efficiency of energy production by simultaneously generating electricity and utilizing the resulting waste heat for heating or cooling applications. Cogeneration plants offer a sustainable solution to energy demands, reducing both environmental impact and operational costs.
Furthermore, the advent of advanced materials and nanotechnology has opened new frontiers in waste heat utilization. From thermoelectric materials capable of converting heat into electricity to nanostructured coatings enhancing heat transfer efficiency, these cutting-edge innovations push the boundaries of what is achievable in the realm of thermodynamics.
Yet, amidst the progress and innovation, challenges persist. The complex interplay of factors such as temperature gradients, heat fluxes, and material properties demands a multidisciplinary approach to waste heat utilization. Moreover, economic viability and scalability remain key considerations in implementing these technologies on a global scale.
As we navigate the mysteries of heat transfer and delve deeper into the realm of waste heat utilization, one thing becomes abundantly clear – the potential is vast, and the possibilities are endless. From reducing carbon emissions to enhancing energy security, the journey towards harnessing waste heat is not just a scientific endeavor but a transformative quest towards a more sustainable future.
At the end, waste heat utilization stands as a testament to the ingenuity of human innovation and the timeless principles of thermodynamics. Through relentless exploration and experimentation, we unravel the enigma of heat transfer, unlocking the latent energy that surrounds us and paving the way towards a brighter tomorrow.
Conclusion:-
The enigmatic realm of thermodynamics, with its intricate laws governing heat transfer, unveils a captivating saga of energy exchange and transformation. Through our exploration, we've delved into the mysteries that lie within this fascinating domain.
From the foundational principles of the first law, which asserts the conservation of energy, to the profound insights of the second law, revealing the inevitable march towards entropy, thermodynamics paints a vivid portrait of the universe's workings.
We've journeyed through the landscapes of heat conduction, convection, and radiation, witnessing the dance of molecules as they transmit energy in various forms. Each mechanism, from the intimate touch of molecules in conduction to the fluid motion of currents in convection, adds layers to the intricate tapestry of heat transfer.
Yet, amidst the complexity, we've uncovered patterns and principles that underpin the chaotic dance of particles. Through experimentation and observation, scientists have illuminated the pathways through which energy flows, driving the processes that sustain life and shape the cosmos.
As we conclude our expedition into the depths of thermodynamics, we are reminded of the profound implications of these laws on our understanding of the universe. From the efficiency of engines to the dynamics of climate systems, the principles of heat transfer permeate every facet of our existence.
While many mysteries remain, and new frontiers beckon, our exploration of thermodynamics has equipped us with a deeper appreciation for the interconnectedness of energy and matter. In our quest to unravel the secrets of the universe, the enigma of heat transfer will continue to inspire wonder and drive the relentless pursuit of knowledge.


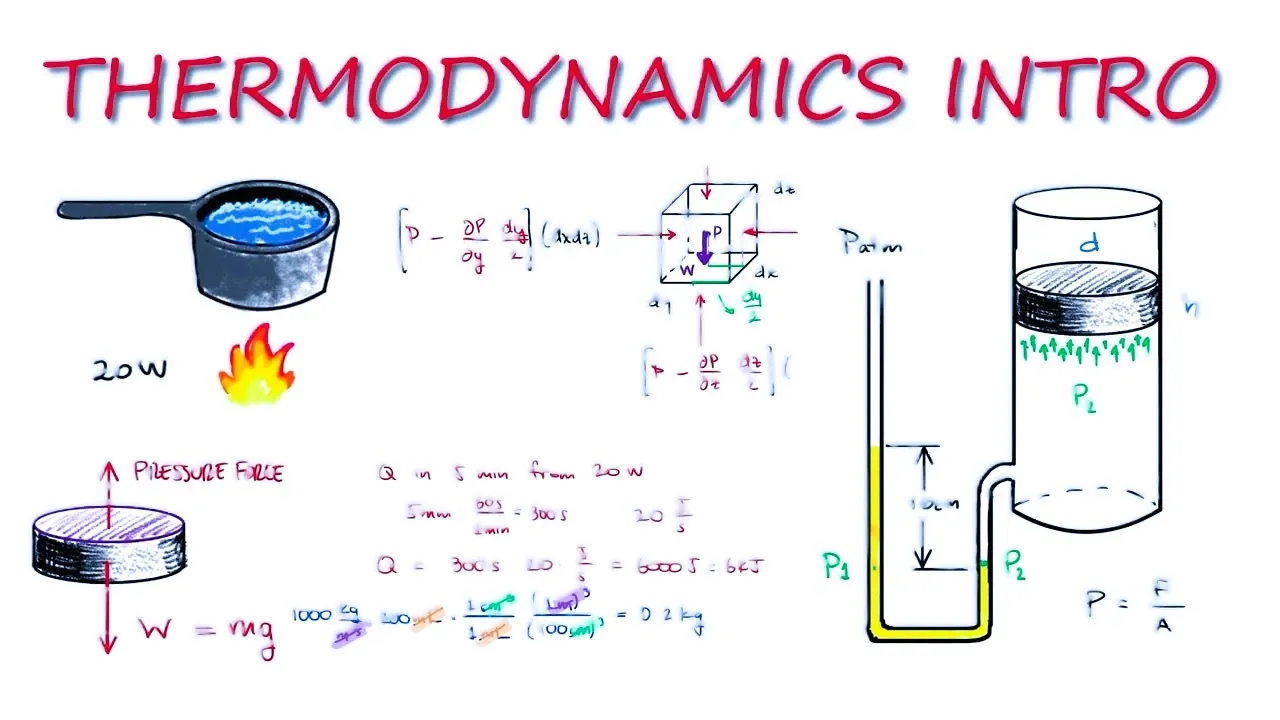








.webp)

0 Comments